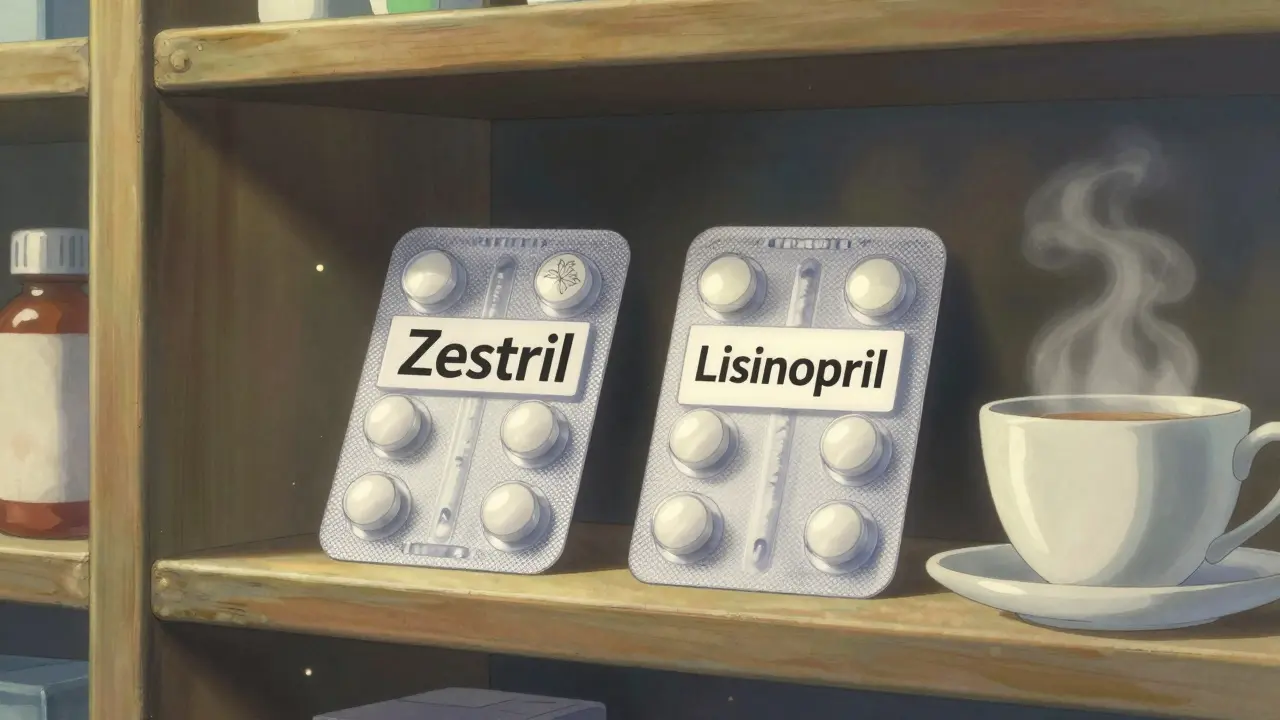
Have you ever picked up a prescription and noticed the pill looks exactly the same as your usual brand-name drug-but the box says something completely different? Maybe it’s labeled "Lisinopril" instead of "Zestril," or "Metformin" instead of "Glucophage." You might wonder: Is this the same thing? Is it safe? And why is it cheaper?
The answer is simpler than you think. These are authorized generics-and they are, in every meaningful way, the exact same medication as the brand-name version you’ve been taking. No differences in how they work. No differences in how they’re made. Just a different label.
What Exactly Is an Authorized Generic?
An authorized generic is a brand-name drug sold without the brand name on the package. It’s made by the same company that makes the original drug, using the same factory, the same equipment, and the same formula. The only thing missing is the brand logo, the fancy packaging, and the marketing budget.
The U.S. Food and Drug Administration (FDA) defines it clearly: "An authorized generic is an approved brand name drug that is marketed without the brand name on its label. Other than the fact that it does not have the brand name on its label, it is the exact same drug product as the branded product."
That means every active ingredient, every inactive ingredient-like fillers, dyes, and coatings-is identical. If your brand drug uses cornstarch as a binder, so does the authorized generic. If it has a red dye, the generic has it too. No substitutions. No changes.
This is different from traditional generics. Traditional generics must prove they’re "bioequivalent"-meaning they deliver about the same amount of active ingredient into your bloodstream as the brand. But they’re allowed to use different inactive ingredients. And sometimes, those differences matter.
Why Do Authorized Generics Exist?
Authorized generics came about because of the Hatch-Waxman Act of 1984. That law created a faster, cheaper way for generic drugs to enter the market after a brand’s patent expires. But brand-name companies didn’t want to lose all their profits overnight.
So they started making their own generics. Not as a separate product. Not as a knockoff. But as the exact same drug, just without the brand name. They’d launch it right when the patent expired, undercutting other generic makers by offering the same pill at a lower price.
It’s a smart business move. Instead of letting a competitor steal their market, they keep control. And they still make money-just less than before. It also gives patients a lower-cost option without switching to a drug they might not trust.
How Are Authorized Generics Different From Traditional Generics?
Here’s the key difference:
- Authorized generic: Made by the brand company under the same FDA approval (NDA). Same active ingredients. Same inactive ingredients. Same manufacturing process. Same pill.
- Traditional generic: Made by a different company under a separate approval (ANDA). Same active ingredient, but inactive ingredients can vary. Made in a different factory, sometimes with different equipment.
For most people, this doesn’t matter. But for some, it does.
Take someone with a rare allergy to a dye like FD&C Red No. 40. If their brand drug uses that dye, and their traditional generic switches to a different dye-or worse, skips it entirely-they might be fine. But if the generic changes the filler or coating, it could cause a reaction. That’s rare, but it happens.
An authorized generic doesn’t have that risk. It’s the same pill. Same dye. Same coating. Same everything.
And here’s something most people don’t know: authorized generics don’t even show up in the FDA’s Orange Book, where traditional generics are listed with therapeutic equivalence ratings. Why? Because they’re not separate drugs-they’re the brand drug under a different label.

Do Authorized Generics Cost Less?
Yes-but not always as much as you’d expect.
Traditional generics usually cost 80-85% less than brand-name drugs. Authorized generics? They’re often priced between the two. Maybe 30-50% cheaper than the brand, but still more than a traditional generic.
Why? Because the brand company still owns the authorized generic. They’re not trying to win a price war with other generics. They’re just trying to keep you from switching to someone else’s version.
That means your out-of-pocket cost might be lower than the brand-but not as low as you’d get with a regular generic. Some insurance plans treat authorized generics like traditional generics. Others treat them closer to the brand. It depends on your plan’s formulary.
GoodRx data from 2023 shows that for some popular drugs, the authorized generic costs $15 for a 30-day supply, while the brand is $80. The traditional generic? $8. So yes, you save-but not always as much as you think.
Are Authorized Generics Safe?
Yes. Absolutely.
The FDA says they’re therapeutically equivalent to the brand. That means they work the same way in your body. They’re absorbed the same. They last the same. They have the same side effects.
A 2018 study published in the National Center for Biotechnology Information followed over 5,000 patients who switched from brand drugs to either traditional generics or authorized generics. The results? No meaningful difference in hospital visits, emergency room trips, or how often people stopped taking their meds.
One small finding: patients on authorized generics had slightly higher emergency room visits than those on traditional generics. But researchers think that’s because authorized generics were often used by patients who had already switched from the brand-and those patients were more likely to have complex health issues.
Dr. Choudhry from Harvard Health put it simply: "Authorized generics have no variation in active ingredient concentration. They are the exact same drug."
For patients who’ve had bad reactions to traditional generics-especially those with allergies, autoimmune conditions, or sensitive digestive systems-authorized generics are often the safest choice.
What Do Pharmacists Say?
Pharmacists see this every day. They’re the ones handing you the pill bottle and explaining why your prescription looks different.
According to Pharmacy Times (2023), about 30% of patients question the switch to an authorized generic. They think it’s a different drug. They worry it won’t work. They’ve heard rumors about generics being "weaker" or "made in China."
That’s why pharmacists are trained to explain: "This is the exact same pill your doctor prescribed. It’s just not labeled with the brand name."
Some patients even ask for authorized generics by name. One patient on the American Academy of Allergy, Asthma & Immunology forum wrote: "I’ve been using the authorized generic of Xyzal for two years with identical results. No itching, no drowsiness, no difference."
But here’s the catch: pharmacists can’t always substitute an authorized generic unless your doctor allows it. If your prescription says "DAW" (Dispense As Written), they can’t switch it without calling your doctor. That’s why it’s important to know what you’re getting.

How to Spot an Authorized Generic
It’s not always obvious. Here’s how to tell:
- Check the label. Does it say the brand name? If not, it’s either a traditional generic or an authorized generic.
- Look at the manufacturer. If it’s the same company that makes the brand drug (like Pfizer, AbbVie, or Merck), it’s likely an authorized generic.
- Ask your pharmacist. They can tell you if it’s an authorized generic or a traditional one.
- Search the FDA’s website. They list authorized generics separately from traditional generics.
Some authorized generics even look identical to the brand pill-same color, same shape, same imprint code. That’s because they’re made on the same line.
When Should You Choose an Authorized Generic?
You should consider an authorized generic if:
- You’ve had side effects or allergic reactions to traditional generics
- You’re on a medication where tiny changes matter-like seizure drugs, thyroid meds, or blood thinners
- Your insurance covers it at the same cost as a traditional generic
- You want peace of mind and don’t want to risk switching ingredients
But if you’ve been taking a traditional generic for years without issues, there’s no need to switch. The FDA says they’re safe and effective for most people.
The Bigger Picture: Why This Matters
Authorized generics are a quiet revolution in how drugs are sold. They prove that the brand name doesn’t make the drug better. The pill is the pill. The science doesn’t care what it’s called.
But they also expose a flaw in the system: companies can use authorized generics to delay real competition. By offering their own version at a slightly lower price, they keep patients from switching to cheaper, independent generics.
That’s why some lawmakers are pushing for more transparency. The FDA is considering requiring authorized generics to be listed in the Orange Book with pricing data. That way, patients and insurers can see exactly what they’re paying for.
For now, the choice is yours. And the truth is simple: if you’re looking for the exact same drug as your brand-name prescription, an authorized generic is the closest thing you’ll find.
It’s not a compromise. It’s not a downgrade. It’s the same medication-just without the brand name on the bottle.
Are authorized generics as effective as brand-name drugs?
Yes. Authorized generics are made by the same company, in the same factory, with the exact same ingredients and formulation as the brand-name drug. The FDA considers them therapeutically equivalent. There is no difference in how they work in your body.
Why are authorized generics sometimes more expensive than traditional generics?
Because they’re sold by the brand-name manufacturer or its affiliate. They’re not competing on price with independent generic companies. Instead, they’re designed to keep patients from switching to cheaper alternatives. As a result, they’re often priced higher than traditional generics-sometimes only 30-50% below the brand, not 80-85%.
Can I ask my pharmacist for an authorized generic?
Yes. You can ask your pharmacist if an authorized generic is available for your prescription. They can check the manufacturer and label to confirm. If your doctor hasn’t specified "Dispense As Written," they can usually substitute it unless your insurance blocks it.
Do authorized generics have the same inactive ingredients as brand-name drugs?
Yes. Unlike traditional generics, which can use different fillers, dyes, or coatings, authorized generics must use the exact same inactive ingredients as the brand-name drug. This makes them a safer option for people with allergies or sensitivities to certain additives.
Are authorized generics listed in the FDA’s Orange Book?
No. Authorized generics are not listed in the FDA’s Orange Book because they are marketed under the brand drug’s original approval (NDA), not a separate generic application (ANDA). Traditional generics are listed there with therapeutic equivalence ratings; authorized generics are not.


14 Comments
Wilton Holliday-22 December 2025
Just switched to the authorized generic for my blood pressure med last month and honestly? No difference at all. Same pill, same results. Saved me like $50 a month. 🙌
Raja P-24 December 2025
Interesting! In India, we don’t really have this concept - generics are just generics. But I get what you mean. If the pill looks and works the same, why pay more? Makes sense.
Joseph Manuel-24 December 2025
While the FDA claims equivalence, the regulatory oversight of manufacturing processes for authorized generics is not transparent. There’s no public audit trail for excipient sourcing or batch consistency. This is a marketing illusion dressed as consumer savings.
Harsh Khandelwal-25 December 2025
Bro, authorized generics are just Big Pharma’s way of saying, "Hey, we still own your meds even after the patent died." They’re not saving you money-they’re keeping you hooked. The real generics? Those are the ones made in Bangladesh. You think your $8 pill is safe? Lol.
Andy Grace-26 December 2025
I’ve used both. The authorized generic felt… familiar. Like the same comfort in a different wrapper. Not a huge price drop, but I’ll take peace of mind over a few bucks.
Delilah Rose-27 December 2025
You know, I think this whole thing highlights how deeply we’re conditioned to equate brand names with quality-like if it doesn’t have a fancy logo, it’s somehow less legitimate, even though chemically it’s identical. I used to panic when my pill changed color, but after reading up on this, I realized I was just scared of change, not the medication itself. It’s kind of a weird psychological thing, isn’t it? We’ll pay extra for the same thing just because the box looks nicer. I’ve started asking for authorized generics now, and honestly, it’s been a small but meaningful shift in how I think about healthcare and consumerism.
Spencer Garcia-29 December 2025
Same pill. Same factory. Just cheaper. Ask your pharmacist.
Lindsey Kidd-30 December 2025
My mom’s on a thyroid med and she switched to the authorized generic after a bad reaction to a regular one. She cried happy tears because her energy came back. No more brain fog. 🥹 Same exact pill. Just no logo. So proud of her for asking questions!
Austin LeBlanc-31 December 2025
Wow, so you’re just gonna trust Big Pharma to give you their own drug cheaper? That’s like trusting the fox to guard the henhouse. They’re not doing this for you-they’re doing it to stop real competition. You’re being played.
Charles Barry- 2 January 2026
Authorized generics? More like corporate manipulation. The FDA is in bed with pharma. They don’t list these in the Orange Book because they don’t want you to know they’re just rebranded brand-name drugs. You think this is about savings? It’s about control. They want you to think you’re getting a deal while they still own your prescription. Wake up.
Lu Jelonek- 3 January 2026
In my family, we’ve always trusted generics. But after my aunt had a reaction to a traditional one, we learned about authorized ones. Now we always ask. It’s not about fear-it’s about knowing your options. Small details matter, especially with chronic meds.
Ademola Madehin- 3 January 2026
OMG I JUST REALIZED MY PILL LOOKS EXACTLY THE SAME AS THE BRAND BUT I’M PAYING HALF?! I’VE BEEN OVERPAYING FOR YEARS 😭😭😭 THIS CHANGES EVERYTHING
suhani mathur- 4 January 2026
So you’re telling me I’ve been paying $80 for Zestril when I could’ve been getting the same pill for $15? And the pharmacist never told me? 🤦♀️ Thanks for the education, OP. I’m calling my pharmacy tomorrow.
Adarsh Dubey- 5 January 2026
Clarification: authorized generics are not merely "the same pill"-they are legally identical to the branded product under the original NDA. The distinction from traditional generics is not merely cosmetic but regulatory and manufacturing. This is a critical nuance often overlooked.