Author: Caspian Whitlock - Page 2
ACE Inhibitors and Potassium-Sparing Diuretics: Understanding the Hyperkalemia Risk
Combining ACE inhibitors with potassium-sparing diuretics can dangerously raise potassium levels, leading to life-threatening heart rhythm problems. Learn who’s at risk, how to spot it early, and what to do if your levels climb.
Hyperpigmentation in Skin of Color: Causes, Treatments, and Sun Protection
Hyperpigmentation in skin of color causes dark patches due to excess melanin. Sun exposure, inflammation, and hormones are key triggers. Effective treatments include sunscreen, topical creams, and addressing root causes. Learn proven management strategies for this common condition.
Citalopram and Escitalopram QT Prolongation Risks: Safe Dose Limits Explained
Citalopram and escitalopram are antidepressants with QT prolongation risks. Learn about dose limits, cardiac effects, and how to stay safe. Regulatory guidelines and practical advice for patients and doctors.
Generic Availability: Why Drug Choices Differ Across Countries
Generic drugs are used worldwide, but availability and pricing vary wildly-from over 90% use in the U.S. to under 20% in Switzerland. Why? Regulation, manufacturing, and policy differences shape what pills you get-and how much you pay.
How to Accurately Document Drug Allergies in Your Medical Records
Accurate documentation of drug allergies in medical records prevents dangerous medication errors. Learn what details to include, how to fix outdated entries, and why vague terms like 'penicillin allergy' can put you at risk.
Noise Exposure Limits: How to Protect Your Hearing at Work and Concerts
Learn the science-backed noise limits that protect your hearing at work and concerts. Discover how to prevent permanent hearing loss with practical steps you can take today.
Anticholinergic Burden in Older Adults: How Common Medications Affect Memory and Thinking
Anticholinergic burden from common medications like Benadryl and bladder pills can cause memory loss and increase dementia risk in older adults. Learn which drugs are dangerous and what safer alternatives exist.
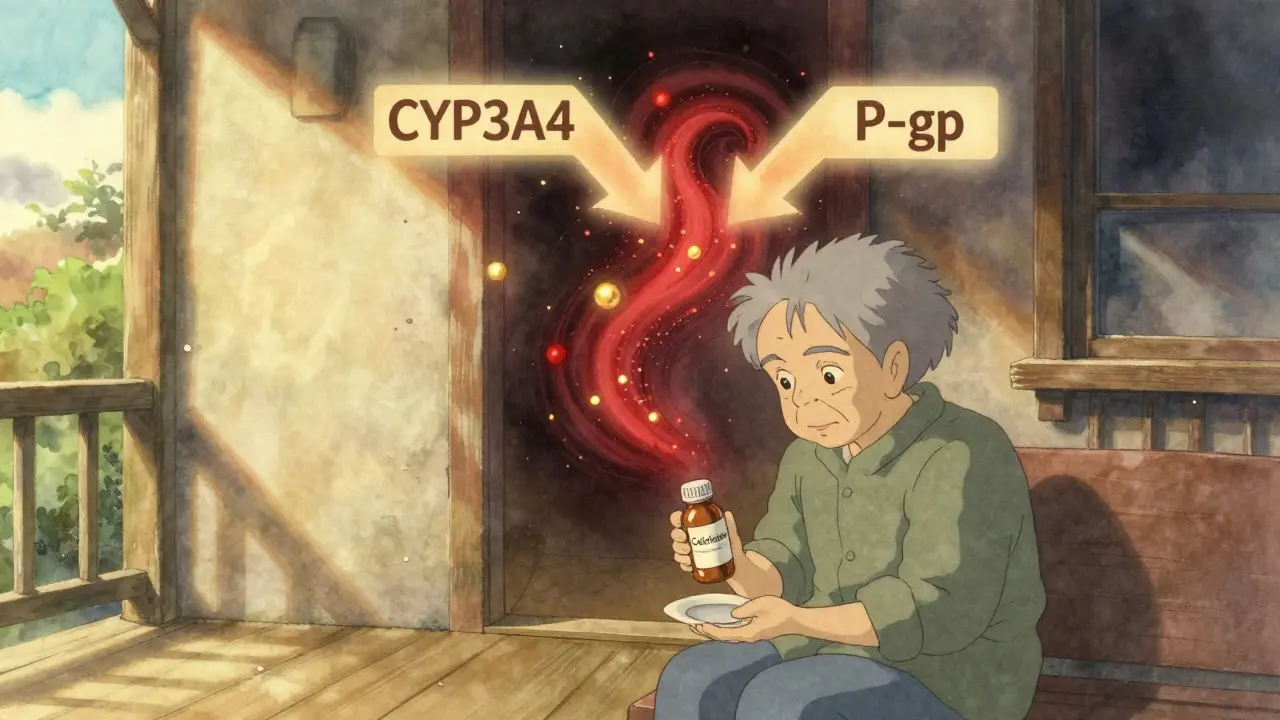
Colchicine and Macrolides: How Together They Can Cause Dangerous Toxicity
Colchicine and macrolides like clarithromycin can cause deadly toxicity when taken together due to dual inhibition of CYP3A4 and P-gp. Azithromycin is a safer alternative. Know the risks and how to prevent them.
Recent Advances in Bioequivalence Testing: Emerging Technologies Driving Faster Generic Drug Approval
Recent advances in bioequivalence testing use AI, virtual platforms, and advanced imaging to speed up generic drug approval. These innovations cut costs, improve accuracy, and expand access to affordable medicines worldwide.
Community Health Presentations: Public Education Resources on Generic Drugs
Community health presentations are helping patients understand that generic drugs are just as safe and effective as brand-name medications - saving billions annually. Learn how FDA-approved generics work, why people still doubt them, and what’s changing in 2025.