Generic Drugs: What They Are, Why They Work, and When to Watch Out
When you hear generic drugs, medications that contain the same active ingredient as brand-name pills but cost far less. Also known as generic medications, they are approved by the FDA to work the same way as the original—but they’re not always the same in every way. Most people assume a generic is just a cheaper copy, and for many drugs, that’s true. But for others—like thyroid meds, blood thinners, or ADHD treatments—even tiny differences in how the body absorbs the drug can cause real side effects. That’s why switching to a generic isn’t always as simple as saving a few dollars.
What most people don’t realize is that inactive ingredients, the fillers, dyes, and binders in pills that don’t treat your condition but help the drug hold together or be absorbed can make a big difference. One person might switch from brand-name Adderall to a generic and suddenly feel jittery or nauseous—not because the active ingredient changed, but because the new pill uses a different coating or filler that irritates their stomach. Same with narrow therapeutic index, a term for drugs where the difference between a safe dose and a harmful one is very small. Warfarin, lithium, and levothyroxine all fall into this category. Even a 5% change in how quickly the body processes the drug can throw your levels off, leading to dangerous side effects. That’s why doctors sometimes advise against switching these unless absolutely necessary.
And it’s not just about the pill itself. The same generic drug made by two different factories might behave differently in your body. Some are made overseas, where regulations vary. Others use cheaper binders that don’t dissolve the same way. That’s why you might feel fine on one generic brand but worse on another—even if the label says the same thing. The FDA says they’re bioequivalent, but bioequivalent doesn’t always mean identical in how you feel. If you’ve ever switched to a generic and noticed new side effects, you’re not imagining it. You’re not alone. And you’re not crazy. The system isn’t perfect.
That’s why the posts below cover real cases, hidden risks, and practical steps you can take. You’ll find stories of people who felt worse after switching, guides on how to spot dangerous fillers, and advice on when to push back and stick with the brand. We’ll show you how to talk to your pharmacist, what to look for on the label, and which drugs are safest to switch—and which ones you should think twice about. This isn’t about fear. It’s about knowing what’s really in your medicine, so you can make smart choices without overpaying.
Generic vs. Brand Name Drugs: What You Really Need to Know About Bioequivalence and Cost Savings
Generic drugs are just as safe and effective as brand names, saving patients up to 85% on medication costs. Learn how bioequivalence works, when to be cautious, and why generics are the smart choice for most prescriptions.
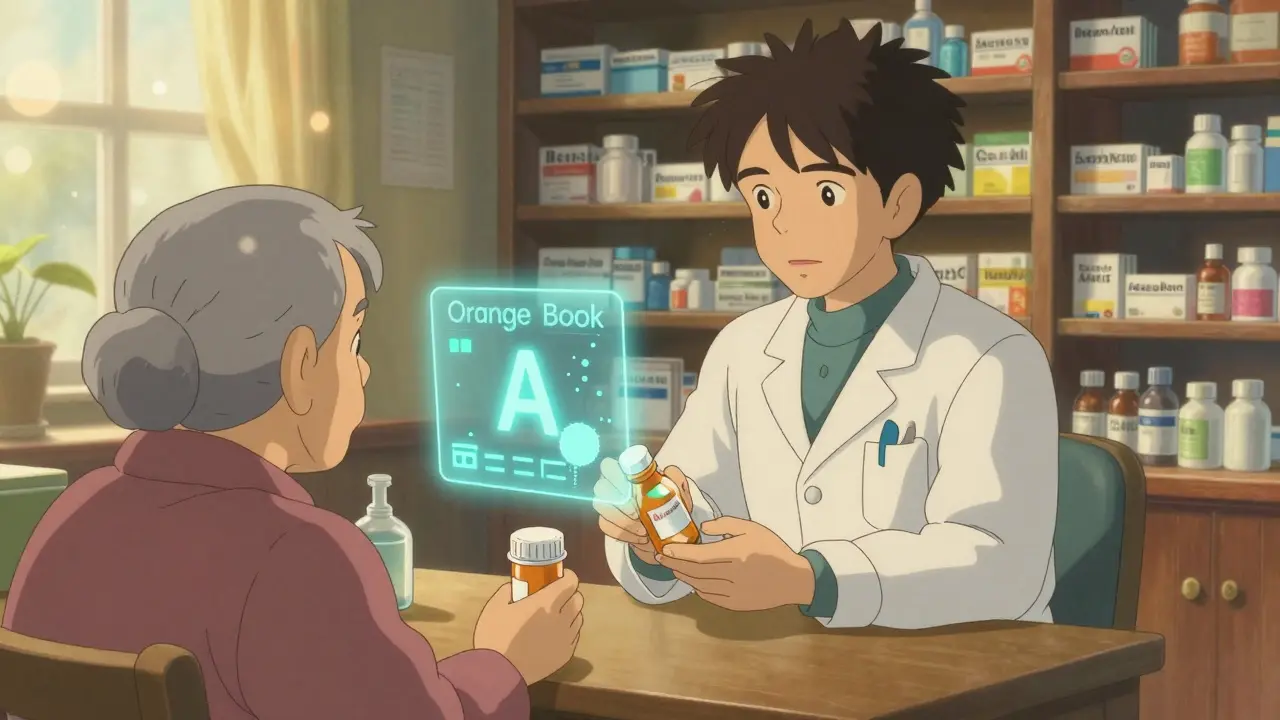
How Pharmacists Verify Generic Equivalence: Practice Standards
Pharmacists use the FDA's Orange Book to verify that generic drugs are therapeutically equivalent to brand-name medications. This system ensures safe, legal substitutions based on strict pharmaceutical and bioequivalence standards.
IVIVC and Waivers: How In Vitro Methods Are Replacing In Vivo Bioequivalence Testing
IVIVC uses lab-based dissolution tests to predict how drugs behave in the body, replacing costly human bioequivalence trials. Learn how it works, why most submissions fail, and why it's the future of generic drug approval.
Placebo Effect with Generics: Why Perception Shapes Medication Outcomes
Why do some people feel generics don't work as well as brand-name drugs-even when they're chemically identical? The answer lies in perception, not chemistry. This article explores how belief shapes real biological outcomes.
Keeping a Medication Journal: Tracking Your Response to Generic Drugs
Track your body's response to generic medications with a simple journal. Learn what to record, why it matters, and how to use your notes to protect your health when switching brands.
Therapeutic Equivalence Codes (TE Codes) Explained: How Generic Drugs Are Approved and Substituted
Therapeutic Equivalence Codes (TE Codes) tell you which generic drugs are safe to substitute for brand-name versions. Learn how the FDA's Orange Book system works, why it saves billions, and when substitutions might not be ideal.
Doctor Attitudes Toward Generic Drugs: What Providers Really Think
Doctors are key to whether patients accept generic drugs. Many still doubt their effectiveness, despite FDA approval. Learn what drives their attitudes-and how education can change prescribing habits.
Hospital Formularies: How Systems Choose Generic Drugs
Hospital formularies systematically choose generic drugs based on clinical evidence, safety, and cost. Learn how Pharmacy and Therapeutics committees make these decisions, why generics are preferred, and how they impact patient care and hospital budgets.